|
A pivotal study consisting of an acute phase and a long-term phase was done to assess acute and long-term efficacy, safety, and tolerability of VNS Therapy.
Pivotal Study: acute phase
The acute phase of this study was a 12-week, double-blind, randomized study comprised of 235 patients at 21 study sites. All of the 235 patients were implanted with the VNS Therapy system, but the control group did not receive active stimulation. All baseline medications were kept stable for 4 weeks prior to the start of the acute phase and remained stable throughout the acute phase of the study.1
Inclusion criteria
The patients were male and female, 18 to 80 years old, with a current diagnosis of major depressive episode and a history of chronic (≥ 2 years) or recurrent (≥ 4 episodes) depression, with 2 to 6 unsuccessful adequate treatment trials* in current episode, and a 24-item Hamilton Rating Scale for Depression (HAMD24) score ≥ 20.1
In addition:
- Patients with both unipolar and bipolar depression were included.
- Medications taken during the study included heterocyclics/tricyclics, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), and other antidepressants; anticonvulsants; lithium; stimulants; atypical and conventional antipsychotics; and anxiolytics.
- Patients with bipolar depression required an unsuccessful trial of lithium for inclusion.
- An unsuccessful 6-week trial of psychotherapy was also required for inclusion.
Exclusion criteria
The main exclusion criteria were a history of atypical depression, psychotic symptoms, schizophrenia, schizoaffective disorder, delusional disorders, rapid-cycling bipolar disorder, acute suicidality or active substance abuse (within the last 6 months).1
Response rates after 8 weeks' fixed-dose stimulation1
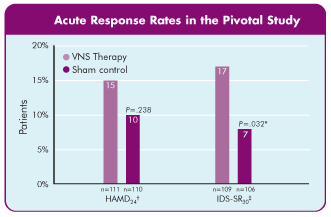
*Significant at the p=.032 level using the last observation carried forward method.
†Hamilton Rating Scale of Depression, 24 items.
‡Inventory of Depressive Symptomatology Self-Report, 30 items
Long-term phase
All patients completing the acute phase received active VNS Therapy in addition to treatment as usual. Medications could be changed during the long-term phase. For clarification, review the 12-Month Response and Remission Rates.
Comparative Study
This long-term, naturalistic, prospective, multicenter study included 127 patients enrolled in 13 sites. 12 of the 13 sites were participating in the Pivotal Study also. All patients received treatment as usual with any available treatment, except VNS Therapy.2
The Comparative Study provides a clinically relevant active treatment control for comparison with the long-term phase of the Pivotal Study. The studies share the following characteristics:2
- Overlapping investigational sites
- Same principal enrollment criteria
- Similar time-frame
- Similar patient baseline characteristics and treatment history
Patient characteristics were similar in the 2 studies2

†All treatments met ATHF (Antidepressant Treatment History Form) criteria for adequate dose, duration and peer reviewed published data from a randomized controlled trial.
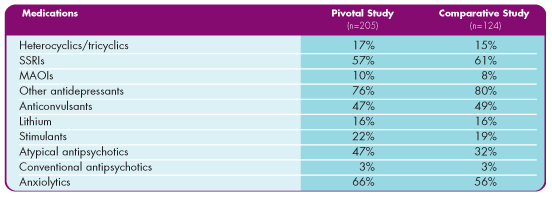
Mood disorder treatments taken during the studies3
Long-term benefits
The primary and secondary analyses comparing patients treated with adjunctive VNS Therapy in the Pivotal Study with patients treated with treatment as usual in the comparative study showed that adjunctive VNS Therapy produces statistically significant greater improvement in depressive symptoms over 12 months of treatment.2

*Inventory of Depressive Symptomatology Self-Report, 30-item.
† Clinical Global Impression-Improvement. Evaluable patient populations; observed data.
‡ Statistically significant. Pivotal Study: adjunctive VNS Therapy.
Comparative Study: active treatment as usual without VNS Therapy.
VNS Therapy provided statistically significant and clinically meaningful improvements at 12 months (HAMD24 improvement; P=.001).2
Clinical benefit increases over time4,5

*“Remission” defined as HAMD24 score ≤ 9 (symptom-free).
†“Response” defined as ≥ 50% reduction in HAMD24 score.
Hear from a physician
"Clinical trials have revealed...that 1 in 3 individuals over the course of a year developed a substantial clinical response. And in fact, roughly half of those achieve a remission, an absence of symptoms."
— Dr. A. John Rush, University of Texas Southwestern Medical Center
Listen to this quote
Click here for prescribing and safety information.
1. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347-354.
2. George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364-373.
3. Data on file. Cyberonics, Inc.
4. Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58:355-363.
5. Physician's Manual. VNS TherapyTM Pulse Model 102 Generator and VNS TherapyTM Pulse Duo Model Generator. Houston, Tex: Cyberonics, Inc; 2006.
|